Start your health career with animal biochemistry

Biochemistry 1 (Animal) BSC103 focuses on the biochemistry of humans and animals. Biochemistry is an integral part of all human and animals health studies. Learn with highly qualified and experienced tutors the basis of life while studying in your own home.
To help you get the most out of the course we initially walk you through the fundamentals of chemistry including atomic structure, the periodic table, molecules, nomenclature, organic chemistry and more before investigating common organic compounds such as carbohydrates, lipids and more.
Lessons cover: introduction to biochemistry, lipids, proteins, enzymes, nucleic acids, thermo regulation, carbohydrate metabolism, absorption, acidity, alkalinity, chemical analysis, and industry applications.
Prerequisite: Some secondary school chemistry will be helpful though it is not essential.
" Clients... that have completed courses with ACS that we have spoken to, have all been extremely happy. Leanne & myself are more than happy with the assistance we received and the prompt attention."
— Dynamic Workforce Solutions
Lesson Structure
There are 10 lessons in this course:
-
Introduction To Biochemistry
-
Basics; atoms, chemical bonds, molecules
-
The Periodic Table
-
Parts of a Molecule
-
Common chemical groups
-
Using these groups
-
Arrangement of atoms in a molecule
-
Chemical Nomenclature
-
Hydrocarbons
-
Aromaticity
-
Organisms and Organic Compounds
-
Biochemical Processes in the cell
-
Lipids and Proteins
-
Carbohydrates; types
-
Hydrolysis
-
Carbohydrate Function
-
Lipids
-
Fatty Acids
-
Triglycerides
-
Phospholipids
-
Terminology
-
Commercially useful fats and lipids
-
Proteins
-
Functional Categorisation of Proteins
-
Proteins in the human diet
-
Enzymes and Hormones
-
Classification of hormones
-
Endocrine Glands
-
Enzyme activation
-
Enzyme deactivation
-
Digestion
-
Digestive Enzymes
-
Digestive Hormones
-
Enzyme PBL Project
-
Nucleic Acids
-
Scope
-
Nucleotide Structure
-
RNA
-
DNA
-
ATP
-
ADP
-
Thermo-regulation
-
Raising temperature
-
Lowering Temperature
-
Effect of Temperature on Enzymes
-
Sweat Glands
-
Energy Production
-
Individual BMR
-
Fever
-
Carbohydrate Metabolism
-
Glycogenesis
-
Glycogenolysis
-
Gluconeogenesis
-
Hyperglycaemia
-
Hypoglycaemia
-
Carbohydrate Oxidation
-
Glycolysis Citric Acid Cycle
-
Anaerobic Respiration
-
Carbohydrate Storage
-
Absorption of Carbohydrates
-
Carbohydrates in Mammals
-
Comparing Energy Pathways
-
The Urea Cycle
-
Absorption
-
Digestion
-
Digestive Enzymes
-
Chemical Digestion
-
Absorption
-
Peristalsis
-
Gastric, Pancreatic and Intestinal Juices
-
Acidity and Alkalinity
-
What is pH
-
Measuring pH
-
Buffers
-
Animal Acid Base Balance
-
Acidosis and Alkalosis
-
Mammalian Buffer Systems
-
Role of Renal System in Acid Base Balance
-
Chemical Analysis
-
Biochemical Testing
-
Concentration testing
-
Moles and Molarity
-
Chromatography
-
Spectrophotometry
-
Analysis of Biomolecules
-
DNA Composition
-
RNA Composition
-
Protein Composition
-
Titration
-
Biochemical Applications
-
Environmental and Agricultural Testing
-
Medical Science
-
Poisons/Toxins
-
Cell Structure
Aims
-
Identify characteristics of common chemical compounds important in animal and human biochemistry.
-
Explain the characteristics of major biochemical groups, including carbohydrates, lipids, and proteins.
-
Explain the characteristics of chemicals which control biological processes in animals and humans, including enzymes and hormones.
-
Explain the role of nucleic acids in the biology of animals and humans.
-
Explain the role of thermo-regulation in animals and humans.
-
Explain the role of carbohydrate metabolism in animals and humans.
-
Identify the characteristics of acidity and alkalinity in relation to animals and humans.
-
Develop simple chemical analysis skills relevant to testing animals.
-
Identify applications and uses for biochemical processes and products.
What You Will Do
-
Explain the formulae of ten specified chemical compounds commonly found in animals and humans
-
Calculate the percentages of elements contained in two specified chemical compounds
-
Differentiate between characteristics of major groups of biochemicals.
-
Identify differences between monosaccharides
-
Differentiate between plant and animal/human biochemistry, with three examples of biochemical processes unique to eachand polysaccharides
-
Differentiate between a fat and an oil
-
Explain the characteristics of a specified protein formula
-
Compare two fibrous proteins with two globular proteins
-
Explain the functions of carbohydrates in animals/humans
-
Explain two commercial applications for lipids in the learners chosen industry.
-
Explain two commercial applications for proteins in the learner’s industry
-
Explain two commercial applications for carbohydrates in the industry the learner’s industry
-
Distinguish between an enzyme and a hormone
-
Explain how one specific enzyme functions in an animal/human
-
Explain how one specific hormone functions in an animal/human
-
Explain the relevance of hormones to the learner’s chosen industry sector
-
Explain the relevance of enzymes to the learner’s chosen industry sector
-
Explain the importance of RNA in animals/humans
-
Explain the importance of DNA in animals/humans
-
Describe the biological and chemical differences between RNA and DNA
-
Explain the role of ATP in providing energy for various cellular activities
-
Explain the mechanisms of body heat production in animals/humans
-
Describe the homeostatic processes which regulate body temperature
-
Explain the mechanisms of body heat loss in animals/humans
-
List the main biochemical processes involved in animal/human carbohydrate metabolism
-
Explain glycolysis, including the sequence of chemical reactions involved
-
Explain the Krebs cycle, including the sequence of chemical reactions involved
-
Explain the electron transport chain, including the sequence of chemical reactions involved
-
Explain differences in animal/human carbohydrate metabolism for a specified situation
-
Explain the processes occurring during the absorptive (fed) state
-
Explain the processes occurring during the post absorptive (fasting) state Describe three chemical buffering effects
-
Explain the role of pH in the control of respiration
-
Explain the importance and methods of pH control of human blood
-
Identify factors involved in controlling acidity and alkalinity in a specific case study
-
Compare a chemical test kits (eg. indicator strips) with chemical meters (eg. haemoglobin meter)
-
Explain the practical applications of various analytical techniques in industry
-
Determine the value of analytical techniques used in the learners industry sector
-
Differentiate between chemical toxicity and tolerance
-
Explain the implications of LD50 characteristics of five different chemical substances
-
Explain the implications of half-life characteristics of five different chemical substances
-
List the active toxins in ten poisonous plants or animals which commonly occur your locality
-
Explain the effects of two naturally occurring toxins on the human body
-
Explain the function and use of two different plants as medicines, for humans or animals
-
Determine three different applications for animal tissue culture
Biochemistry Helps us Learn to Understand all Living Things
Chemistry involves study of elements that make up the physical world, and how they interact with each other.
Organic chemistry is the study of chemical compounds containing carbon.
“Bio” means “alive”, therefore biochemistry is the study of the chemistry of biological organisms and how organic chemical compounds react within living cells. In other words, biochemistry is about understanding the chemical reactions that make, break, run and repair our bodies and the components that make it up. Biochemistry’s goal is to understand the chemical basis of biological phenomena.
Biochemistry has its roots in medicine, nutrition, agriculture, and natural products chemistry. It covers many other areas as well, but today it is mostly is concerned with the chemistry of molecules found in and associated with living systems, especially the chemistry of these molecules. Biochemists are always trying to break processes down in order to understand how these work, how molecules are created or destroyed and how they relate and affect each other. With the advent of all the modern equipment and computer systems many biochemists also study intact systems and how each system functions and the other structures or processes that may be affected.
BIOMOLECULES
In addition to carbon, most organic compounds contain hydrogen, and most contain oxygen as well. The atoms of these elements are arranged in various forms to make up most of the dry weight of living organisms. The four main types of biochemical molecules are proteins, carbohydrates, lipids and nucleic acid. Other important molecules for metabolism regulation are hormones and neurotransmitters which are made up of different protein, carbohydrate, lipid and nucleic acid combinations.
Proteins
Proteins are large and complex molecules made from chains of amino acids, much like beads on a necklace. A protein would be like several necklaces connected together and organized showing a well defined compact structure. Proteins include enzymes and hemoglobin. There are 20 standard amino acids which can be combined in different order, number and structure to make an array of different proteins. Many proteins also have non-amino acid molecules added to them, such as lipid or saccharide chains.
All proteins contain nitrogen, and that is a very important characteristic concerning the effect of proteins on health. Proteins are essential to the structure and function of all living cells and viruses. Many proteins are enzymes or subunits of enzymes. Enzymes are required to catalyse most biochemical reactions (a catalyst speeds up a reaction but is not actually used up or altered by the reaction) Other proteins play structural or mechanical roles, such as those that form the structural filaments (collectively known as the cytoskeleton) that give shape and strength to a cells. Other functions that are filled by proteins include immune response (antibodies are proteins) and the DNA stability and structure (histones) and membrane transport (form channels in cell membranes which allow hydrophilic molecules to pass through).
Proteins are typically not multi skilled, they are designed to perform a specific function perfectly and no other function. Some proteins will perform similar functions and will work together, some, such as enzymes are often specialised for a single reaction.
Carbohydrates
Carbohydrates are what we commonly refer to as ‘sugars’. They can be very simple individual molecules, known as monosaccharides up to massive, complex carbohydrates known as polysaccharides. Glucose is an example of a simple sugar, starch and cellulose are complex polysaccharides.
Carbohydrates have the general molecular formula CH2O, and thus were once thought to represent "hydrated carbon". However, the arrangement of atoms in carbohydrates has little to do with water molecules. Carbohydrates or saccharides are the most abundant class of biological molecules. Starch and cellulose are other two common carbohydrates. Both are macromolecules with molecular weights in the hundreds of thousands. Both are polymers (hence "polysaccharides"); that is, each is built from repeating units, monomers, much as a chain is built from its links. The monomers of both starch and cellulose are the same: units of the sugar glucose.
All carbohydrates are made up like this, by linked units of simple sugar monomers (units). They have a ‘backbone’ of carbon molecules, from which –H or –OH branch out.
They are normally broken down into several major classifications of carbohydrates including:
Monosaccharides:
- the basic unit (mono=one). They are made up of 3 to 6 carbon molecules linked together, and with hydrogen and oxygen. They cannot be further broken down.
- Glucose and Ribose
Disaccharides:
- made of two units (di=two). They can be broken down into their monosaccharide constituents
- Sucrose, lactose, maltose
Oligosaccharides:
-
made up with between 3 and 10 monosaccharides.
Polysaccharides:
- more than 10 monosaccharide units
Differences between the saccharides occur with different molecular bonds between the constituent atoms. Carbohydrates have many functions, they are metabolised for energy, added to other molecules to allow them to perform their specific function or achieve their final conformation (shape). They are components of structural and transport molecules, nucleic acids and a variety of other biomolecules.
Lipids
Lipids are basic components of cell membranes and are also used for the storage of energy (fats). All lipids are hydrophobic, meaning they cannot dissolve in water. If you have a cup of water and you add three drops of oil, they will immediately gravitate toward each other and join up. This group of molecules includes fats and oils, waxes, phospholipids, steroids (like cholesterol), and some other related compounds that are part of hormones and neurotransmitters.
Cellular membranes are organized assemblies of lipids. These are not however impermeable, rather they regulate the composition of the intracellular components as well as the flow of the nutrients, waste products, ions, etc through the membrane. Hydrophilic molecules, such as lipids can pass directly through lipid bilayers, and this is the method of action of steroidal drugs. Protein channels in the membranes form pores through which other molecules can pass, but often only in conjunction with a gradient or in exchange for a different molecule. Fat tissue, known more correctly as adipose tissue is the site of storage for excess lipids in the body. When there is a gross excess, fatty deposits will form in and on the organs, as well as in the arteries, with serious health implications. Fat tissue also serves as an endocrine (hormone producing) organ and many hormones contain lipid molecules.
Nucleic Acids
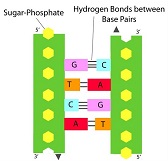
Nucleic acids are contained in the nucleus of the cell. They are the genetic material of all cells and of most viruses. They provide a genetic ‘blueprint’ for the organism and are a macromolecule. There are two main types of genetic material in a cell, DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). When a new protein is needed within a cell, the chromatin (condensed double stranded DNA structure) opens up to the gene that codes for the protein. The gene is the copied to give a new molecule known as RNA (a single strand) in a process known as transcription. The RNA is then used as a template, directing the order of the amino acids to build the new protein, in a process known as translation. The protein then goes through a process of modification, with sugars or lipids or other molecules added to give it its final structure and features required to perform its function.
Nucleic acids are made of nucleotides. Nucleic acids have two main parts - the backbone of a nucleic acid is made of alternating pentose sugar and phosphate molecules bonded together in a long chain. Each of the sugar groups in the backbone is attached to a third type of molecule called a nucleotide base. There are four different bases, adenine, guanine, thymine (replaced by uracil in RNA) and cytosine, known as A, G, T, U and C respectively. Different combinations and sequences of the 4 DNA bases makes up all our genes and is our individual genetic code. No two people have exactly the same genetic code, unless they are identical twins/triplets etc.
Other than the genetic material, nucleic acids are also components of other biomolecules, including ATP (energy), ribozymes and some enzymes and co-enzymes such as Co-enzymeA, NAD and FAD that are important in energy production. The basic two structures of nucleotides are purine (adenosine and guanine) and pyrimidine (cytosine, thymine and uracil).
Vitamins and Co-enzymes
Vitamins are essential biomolecules that are cofactors or coenzymes for the functioning of many enzymes. Often, only trace levels are required, but a shortage can result in disease or death. A common distinction of vitamins is whether they are water soluble or fat soluble compounds.
Fat soluble vitamins are vitamins A, D, E and K. They are dissolved in lipids, and that is another reason why lipids are necessary in food. Because they are dissolved in lipids, they tend to accumulate in the body. Water soluble vitamins, on the other hand, are easily eliminated with urine and other liquid secretions of the body, therefore it is easier to be deficient in these.
Coenzymes are low molecular weight molecules that provide unique chemical functionalities for certain enzyme/coenzyme complexes. They may act as carriers of specific functional groups. They can provide chemically reactive groups that the common twenty amino acid side chains cannot provide. They may also alter the conformation of an enzyme to make it active. Coenzymes are usually modified in the course of a reaction, and subsequently chemically regenerated back to their useful active form. Thus, this recycling of coenzymes means that only small concentrations are required. All the water soluble vitamins (with the exception of vitamin C) are coenzymes or precursors of coenzymes.
Hormones and Neurotransmitters
Hormones and neurotransmitters are communication molecules. They are used to communicate one part of the body with another. Neurotransmitters function in the nervous system while hormones allow communication between cells, often over long distances as the hormone is carried through the bloodstream. The nervous system can monitor hormone levels and react accordingly, stimulating greater production and release of hormones, responding to the hormone levels functionally or providing negative feedback to slow or stop the hormone release.
Hormones are synthesised by the endocrine organs which include the parathyroid, thyroid, pancreas, pituitary gland, adipose tissue and reproductive organs. Neurotransmitters are substances that are synthesized by neurons in order to communicate with other neurons. Their targets are neighbouring neural cells. Their main function is to produce change in the receiving neuron (postsynaptic neuron), which in turns produces a follow on effect with the next neuron and at the end of the chain an effect in a certain part of the body, such as muscle movement, pain feeling, emotions and thoughts, etc, or when carried to the brain, the signal provides sensory information which the brain then responds back to.
WHY STUDY THIS COURSE?
- Fill a gap in your knowledge of chemistry (perhaps you missed it at school or have just never retained what you studied)
- Gain a fundamental understanding so you can manage your own nutrition and eating better
- Understand nutrition and broader chemistry of animals - pets, livestock, wildlife
- Improve your career opportunities by better understanding the inner workings of both humans and animals
